Principal Investigator
At a Glance
When carbon dioxide (CO2 ) is injected into highly reactive rocks like basalts, the injected CO2 will react quickly to form new carbonate rock. This is the most stable form of geological carbon storage. The Celia group built a computer simulation tool to study this process across a range of spatial scales. Small-scale injections show fast reaction rates, on the order of months to years that are consistent with results from small-scale field experiments. However, at the large spatial scales associated with practical industrial-scale injections, largescale mass transfer limitations lead to much longer time scales for the reactions to proceed, on the order of a century or more. The newly developed computational tool allows these and other issues to be investigated efficiently. Progress in modeling and reliably assessing the potential of carbon sequestration in deep geological formations can help accelerate bp’s efforts in decarbonizing heavy industry, while seeking new energy solutions.
Research Highlight
In carbon capture and storage (CCS), anthropogenic CO2 is injected into deep geologic reservoirs. The injected CO2 needs to remain underground for hundreds to thousands of years. The most secure form of underground carbon storage, called “mineral trapping,” occurs when the injected CO2 reacts with minerals in the subsurface to form carbonate rock that will remain buried underground for a very long time.
A few recent small-scale field tests have shown this kind of trapping to be highly effective. For example, as part of a project called CarbFix, a group in Iceland injected CO2 into basalt formations. They discovered that the injected CO2 had transformed to solid rock over a fairly short time (Matter et al., 2016). In that experiment, the injected CO2 was first mixed with water and the resulting aqueous solution was injected. In another small-scale experiment, a group in the Pacific Northwest of the United States (McGrail et al., 2017) injected separate-phase CO2 into a basalt formation and also reported significant reactions. These examples demonstrate the great potential mineral trapping holds for the permanent storage of CO2 .
Several issues need to be addressed before this method can be adopted in practice. One is the issue of scale: Do the same fast reaction rates apply to more realistic large-scale injections, or are other rate-limiting processes involved at larger scales? Another issue is the characterization of the rock mineralogy and reaction kinetics: How much uncertainty is there in the definition of these reactions? And will the precipitation of new rock mass in the subsurface clog the pore space and render the injection system ineffective?
To answer these and other questions, Ph.D. student Tom Postma built a new computer simulation tool that combines earlier work in the Celia group on modeling large-scale two-phase fluid flow in the subsurface (Nordbotten and Celia, 2006, 2012; Gasda et al., 2011) with a flexible geochemistry solver that accommodates a broad range of mineral reactions. The code maintains the efficiency of the original formulations from Nordbotten and Celia while enhancing mass tracking and including different kinds of geochemical reactions. Details of the methodology have been published recently in Postma et al. (2021, 2022).
One key finding is that scale matters. The researchers ran simulations with realistic injection rates (in the order of one million tonnes of CO2 per year) and varied parameters like porosity, permeability, dip angle, and reactive surface area. Small-scale injections show that large fractions of the injected CO2 react on a time scale of months to years. Large-scale injections, by contrast, show that reactions are limited by the much slower mass transfer between regions containing separate-phase CO2 and those containing only brine. An example of these results is shown in Figure 7.1, where the amount of CO2 stored as carbonate rock is shown as a function of time. Even the fastest reaction cases show century time scales for the reactions to proceed.
Another key finding is that reductions of porosity can take place, with the largest changes found in the vicinity of the injection wells. While this could pose some risk of excessive permeability reduction, early results suggest that the porosity reduction, which is not more than a few percent (that is, reduction of porosity from, say, 15% to 13%), may not be enough to cause pore-space clogging. The actual effect on permeability is especially difficult to assess in basalts, but a first, simple estimate is a reduction of about a factor of 2. This reduction tends to be offset by the reduced resistance to flow associated with CO2.
The most sensitive parameter in these simulations is also the most uncertain: reactive surface area. The researchers have taken a range of values from the literature and included them in a wide range of simulations. The researchers are also in the process of investigating different mineralogies, such as basalts from different parts of the world, to determine what characteristics are most effective for mineral trapping. Upcoming publications will report on these findings.
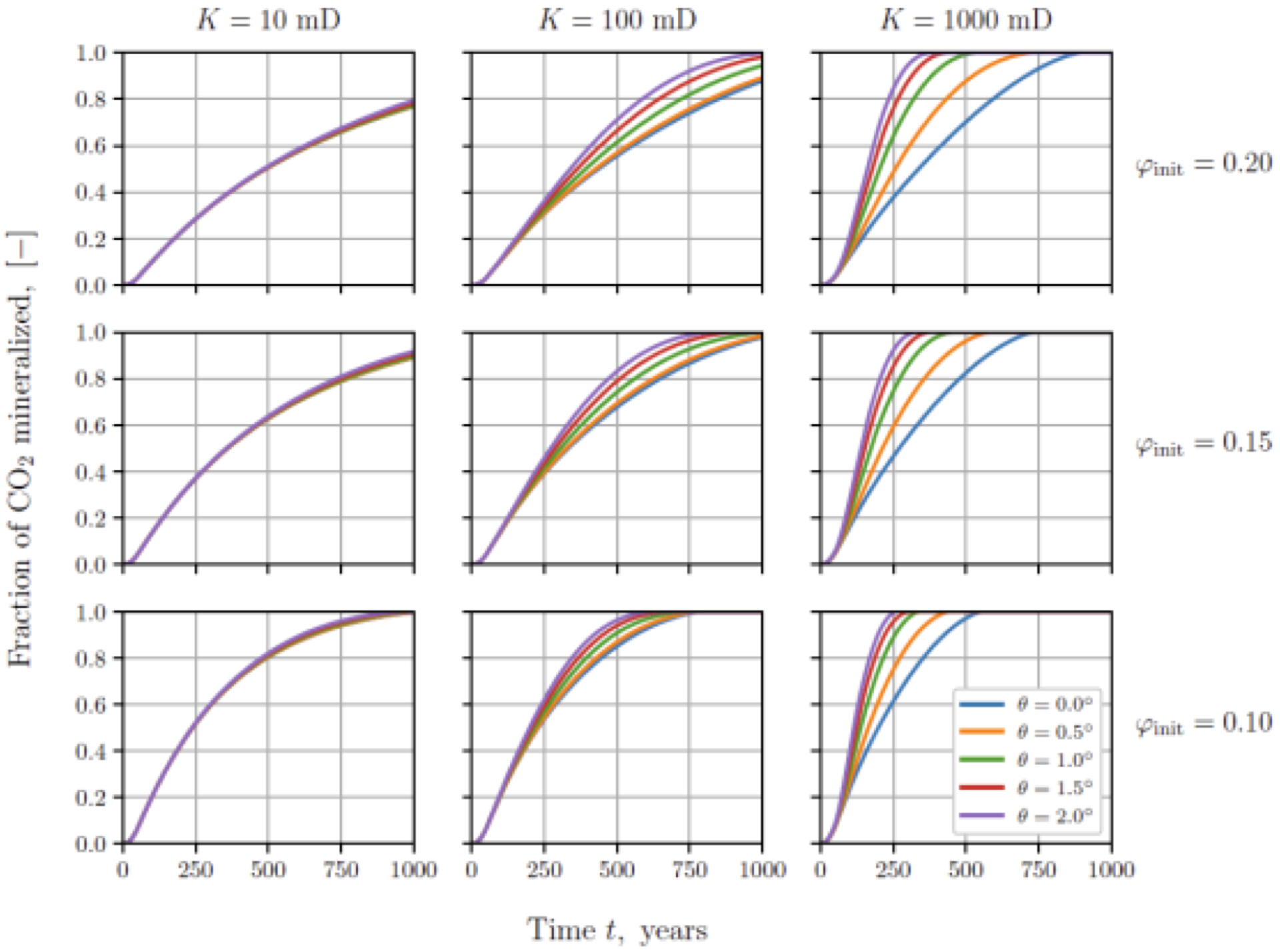
Fraction of the total amount of CO2 trapped as carbonate minerals over time, for injection into reservoirs of varying porosity, permeability, and dip angle.
References
Gasda, S.E., J.M. Nordbotten, and M.A. Celia, 2011. Vertically averaged approaches for CO2 migration with solubility trapping. Water Resources Research 47(5):W05528. (https://doi. org/10.1029/2010WR009075).
Matter, J., M. Stute, et al., 2016. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 352(6291):1312-1314. (https://doi.org/10.1126/science.aad8132).
McGrail, B., H. Schaef, et al., 2017. Field validation of supercritical CO2 reactivity with basalts. Environmental Science and Technology Letters 4(1):6-10. (https://doi.org/10.1021/acs.estlett.6b00387).
Nordbotten, J.M. and M.A. Celia, 2006. Similarity solutions for fluid injection into confined aquifers. Journal of Fluid Mechanics 561, 307-327. (https://doi.org/10.1017/S0022112006000802).
Nordbotten, J.M. and M.A. Celia, 2012. Geological storage of carbon dioxide: Modeling approaches for large-scale simulation, John Wiley and Sons, Hoboken, NJ, USA. (https://doi.org/10.1002/9781118137086).
Postma, T.J.W., K.W. Bandilla, and M.A. Celia, 2021. A Computationally efficient method for field-scale reservoir simulation of CCS in basalt formations. 15th International Conference on Greenhouse Gas Control Technologies, GHGT-15. (http://dx.doi.org/10.2139/ssrn.3828277).
Postma, T.J.W., K.W. Bandilla, C.A. Peters, and M.A. Celia, 2022. Field-scale modeling of CO2 mineral trapping in reactive rocks: A vertically integrated approach. Water Resources Research. (https://doi.org/10.1029/2021WR030626).