Principal Investigator
At a Glance
Enhanced weathering (EW) is a negative-emission technology that holds the potential of alleviating the acidification of soils and natural waters. It is a carbon capture process designed to enhance and accelerate the chemical weathering of natural minerals that, when dissolved, remove carbon dioxide (CO2) from the atmosphere and store it in natural waters. However, because the precise characterization of EW efficiency is still little understood, Porporato’s research group has been working on quantifying the Alkalinization Carbon Capture Efficiency (ACCE) of any mineral dissolution in various natural waters. The findings provide an important step forward in the identification of suitable environmental conditions for EW applications, better quantification of EW carbon sequestration potential, and important context for bp’s plans for natural climate solutions.
Research Highlight
Enhanced weathering (EW) has been gaining attention as a promising geoengineering technology with large potential for CO2 removal and limited technological requirements (Beerling et al., 2020). EW consists of spreading finely ground alkaline minerals in environments where the mineral dissolution might be favored, such as, for example, in the acidic soils of croplands and forests. In these environments, the minerals would also counteract soil acidification and promote biomass growth with the addition of important biological macronutrients. Some of the mineral dissolution products would be transported along the hydrologic cycle to surface freshwaters and, eventually, the ocean, mitigating ocean acidification and stably sequestering atmospheric CO2 for geological timescales (Renforth and Henderson, 2017).
The transfer of CO2 from the atmosphere to water is triggered by alkalinization, or an increase in water alkalinity, which is caused by the mineral dissolution. However, whether CO2 transfer occurs depends on the water’s chemical conditions in which the minerals dissolve. Although this has long been recognized (Hartmann et al., 2013), the conditions that discriminate between efficient and inefficient carbon capture is currently little understood and lacks an objective quantification.
Using principles of aqueous chemistry, Porporato’s group has derived an analytical factor that quantifies the increase in the Dissolved Inorganic Carbon (DIC) in the water solution in response to a small variation in water alkalinity (Alk). The factor, referred to as Alkalinization Carbon Capture Efficiency (ACCE), enables an exact definition of the alkalinization carbon capture efficiency of any mineral. This, in turn, is indicated as ACCEM, which quantifies the amount of CO2 captured per molecule of mineral dissolved (Bertagni and Porporato, submitted). Figure 9.1 shows that ACCE and ACCEM strongly depend on the water pH.
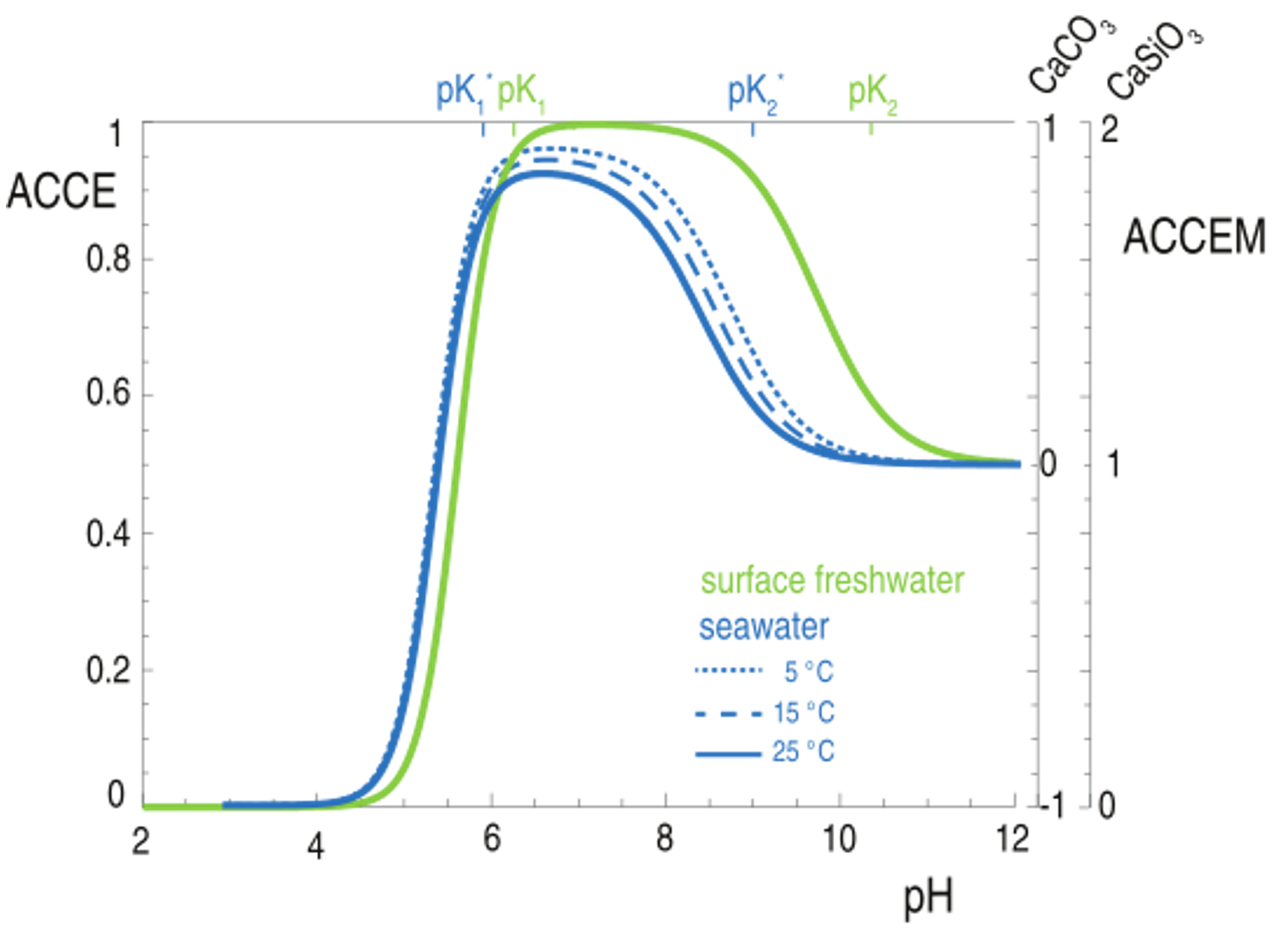
Alkalinization Carbon Capture Efficiency (ACCE) as a function of the water pH for freshwater and seawater in equilibrium with the atmosphere. ACCEM is the ACCE of a specific mineral.
Figure 9.2a reports ACCE evaluation for the world’s topsoils that are sufficiently wet and warm for EW applications. ACCE is maximized in mildly acidic or alkaline areas (e.g., Central Europe, Eastern Asia) and is minimized in very acidic soils (e.g., Scandinavia, central Amazon). Mineral dissolution rates are higher in very acidic soils (Figure 9.2b), resulting in an important trade-off between carbon capture efficiency and enhanced chemical dissolution. This means that, in acidic soils, either the EW application promotes a large alkalinity perturbation that raises the soil water pH to values that are favorable to carbonate formation, or the cations released by the mineral dissolution would need to reach another water solution before transferring any atmospheric CO2 to the water.
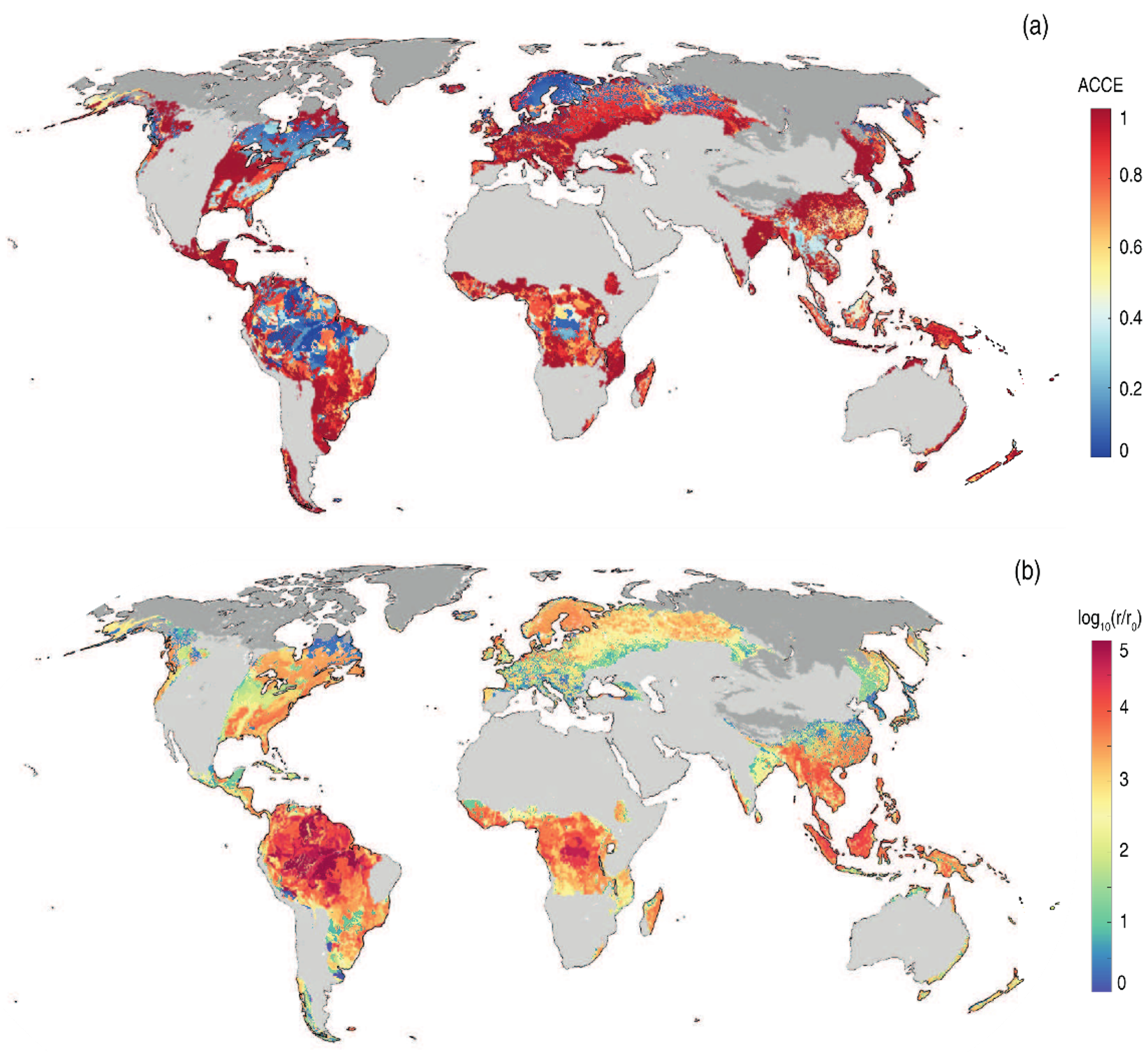
Map of ACCE (a) and mineral dissolution rates (b) in global topsoils (0-30 cm). Dissolution rates account for temperature, moisture and water acidity and are normalized to the minimum. Dark and light grey regions are respectively too cold or too arid for EW applications. The carbon capture efficiency is lower where the dissolution rates are higher (acidic soils).
There is, therefore, a need for further research on the timescales of the mineral cation transport during the hydrological cycle and on the fraction of cations that can be lost to ecosystem uptake or soil adsorption.
References
Beerling, D. J., E.P. Kantzas, M.R. Lomas, et al., 2020. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 583(7815):242-248. (https://doi.org/10.1038/s41586-020-2448-9).
Bertagni, M. B., and A. Porporato. (submitted). The efficiency of enhanced weathering.
Hartmann, J., A.J. West, P. Renforth, et al., 2013. Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Reviews of Geophysics 51(2):113-149. (https://doi.org/10.1002/rog.20004).
Renforth, P., and G. Henderson, 2017. Assessing ocean alkalinity for carbon sequestration. Reviews of Geophysics, 55(3):636-674. (https://doi.org/10.1002/2016RG000533).