Principal Investigators
At a Glance
Portland cement is currently the most common type of cement used in concrete manufacture, but it is a significant source of atmospheric carbon dioxide (CO2) due to the production process. To counter this, White and her group are developing sustainable cements that are alternatives to conventional Portland cement. These cements can reduce CO2 emissions but with limited in-field evidence of proven long-term performance. By understanding the pore structures of these alternative cements, linking pore structure to permeability, and investigating the mechanism of effective additives, the researchers aim to create a predictive phenomenological model that can be used to identify the most suitable alternative cement for a specific environmental application. Reducing concrete emissions in the construction industry would have a large impact on CO2 emissions, which aligns with bp’s ambition of helping the world get to net-zero.
Research Highlight
Hard-to-decarbonize industries such as cement face the daunting task of lowering their CO2 emissions while maintaining product quality and performance. This is particularly challenging for cement and steel, where any change to the chemistry of the material can have long-term, significant ramifications on performance and safety. After water, concrete is the second most consumed resource in the world and is essential to modern infrastructure. However, one key ingredient of concrete, Portland cement powder, currently accounts for approximately 8% of global anthropogenic CO2 emissions. Alternative cements can offer more sustainable options for producing concrete, thus avoiding a significant portion of CO2 emissions in the industry. However, our ability to predict the long-term performance of these more sustainable materials is severely hampered by the time it takes to obtain pore structure data of the binder material that controls ingress of harmful chemicals such as CO2 , sulfate (SO42-), and chlorine (Cl- ) ions.
Recently, White and her group have been focused on reducing CO2 emissions associated with alkali-activated metakaolin (AAMK) cements. They are exploring the possibility of lowering the concentration of the activator – the most carbon intensive component of AAMK – without adversely impacting long-term performance. This involves using a small amount of a cation additive such as calcium hydroxide or reactive magnesium oxide to help offset reduced performance at lower activator concentrations. Permeability quantification measures using beam-bending tests and pore neck size distributions obtained by mercury intrusion porosimetry show that both calcium hydroxide and magnesium oxide can improve the durability of AAMK with low activator concentrations (Figure 10.1). On a per-mole-of-cation basis, calcium hydroxide appears to be more effective than magnesium oxide. However, more magnesium oxide can be added without causing a significant premature decline of mixability, making magnesium oxide a promising additive to ultra-sustainable AAMK systems.
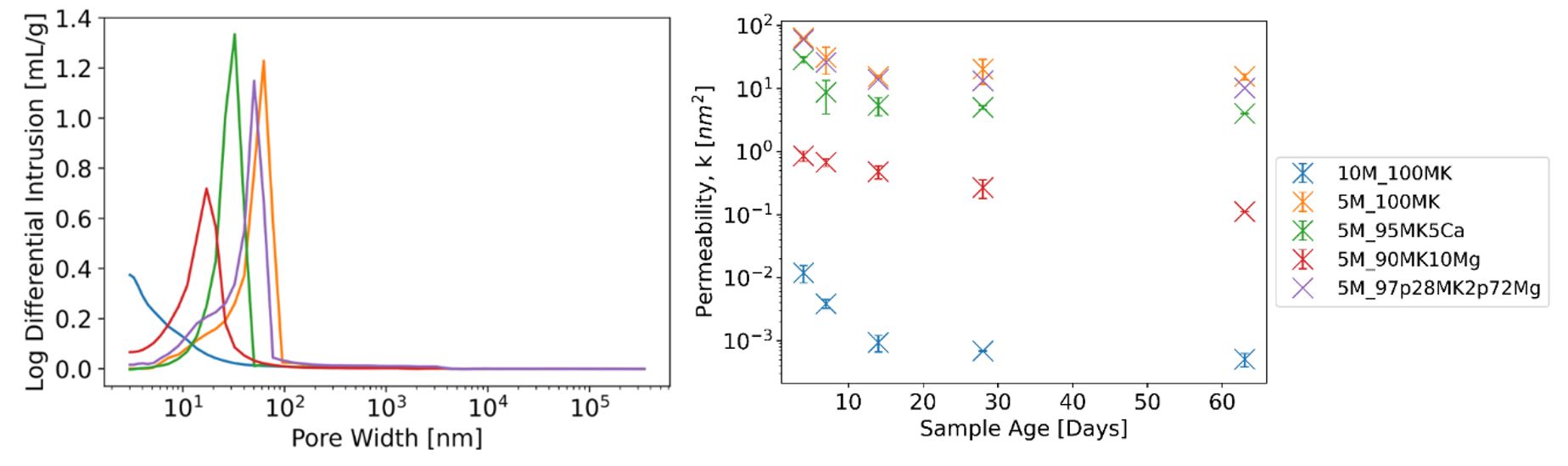
(Left) Mercury intrusion porosity results for AAMK systems with two different activator concentrations and with and without cation additives. (Right) Permeability values as a function of sample age for tested AAMK systems.
White and her group are also studying the characteristics of the ink-bottle pore shape, specifically the inner pore size and the neck pore size. This research uses novel techniques such as neutron grating interferometry and focused ion beam scanning electron microscopy. The hope is that a more comprehensive assessment of cation additives’ effects can be carried out in future studies.
White’s research is also focused on dissecting how cation additives reduce pore sizes and permeability. The researchers used in situ Fourier-transformed infrared spectroscopy (FTIR) and isothermal conduction calorimetry (ICC) to observe alkali activation reactions in real-time (Figure 10.2). Using the FTIR data, the researchers can track changes in the vibration mode associated with the dissolution of metakaolin and the precipitation of gel phases. From ICC, the researchers can measure cumulative heat of each reaction. Preliminary results indicate that both calcium hydroxide and magnesium oxide promote the early dissolution of metakaolin, presumably by increasing the pH of the solvent, thereby resulting in more paste forming. Cation additives also appear to create transient gel phases that may affect the mature paste structure. Additionally, magnesium oxide seems to have some delayed effects on the paste when compared to calcium hydroxide. This difference may explain the two additives’ varied influence on the early-age flow property.
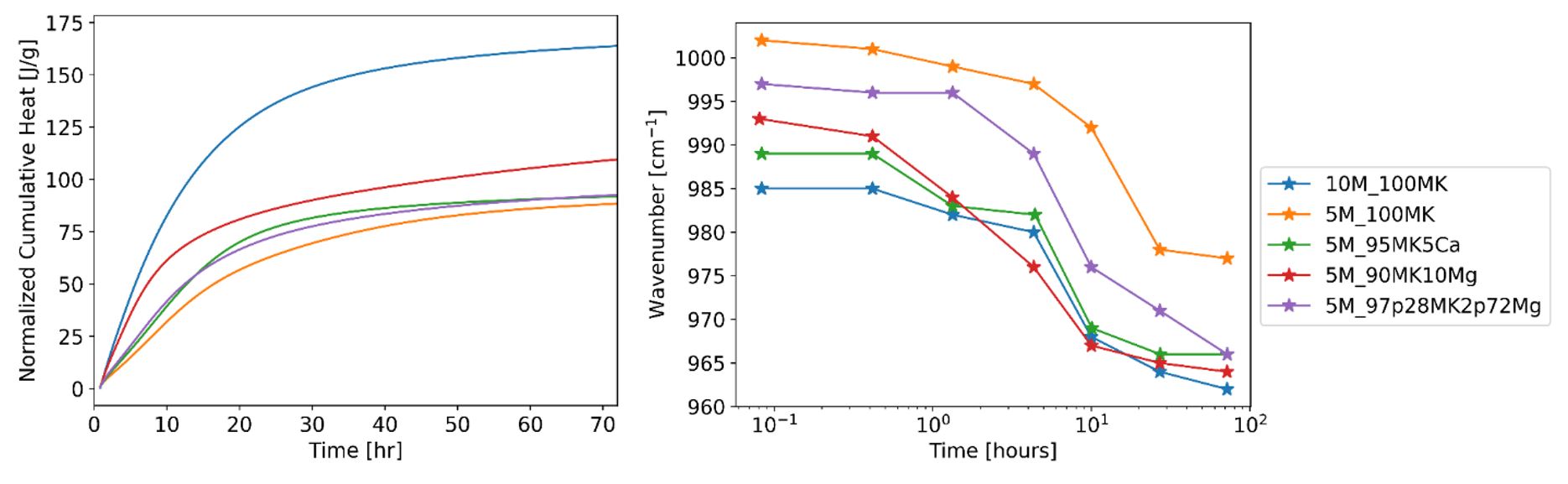
(Left) Cumulative heat flow curves of various AAMK systems, normalized by total sample weights. (Right) Changes in the FTIR vibration mode associated with metakaolin dissolution and gel precipitation.
White and her group are hopeful about achieving the optimization and customization of sustainable cements. They believe that this can be achieved by understanding the ways in which cation additives enhance AAMK systems and establishing a robust structure-property-mechanism relationship for AAMK.
References
Zhang, A. and C.E. White. Effects of calcium hydroxide and magnesium oxide on the pore structure and permeability of alkali-activated metakaolin. In preparation.
Zhang, A, and C.E. White. Mechanistic insights on the role of calcium hydroxide and magnesium oxide in alkali-activation of metakaolin. In preparation.